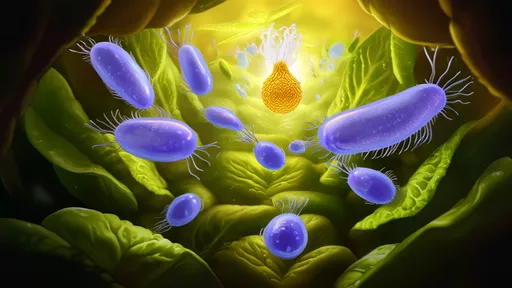
The quiet miracle of a loaf of bread begins not in the oven, but hours earlier, in the humble stillness of a mixing bowl. It starts with flour, water, salt, and a living organism so small it is invisible to the naked eye: yeast. This biological leavening agent is the undisputed architect of bread's most essential characteristic—its airy, tender crumb. The entire process hinges on a fundamental, almost magical phenomenon: the respiration of dough. This is the silent, gaseous ballet where yeast, a single-celled fungus, consumes sugars and exhales carbon dioxide, literally breathing life and structure into what would otherwise be a dense, unpalatable slab.
To understand this transformation, one must first meet the protagonist. Saccharomyces cerevisiae, the baker's and brewer's yeast, is a workhorse of fermentation. When these dormant yeast cells are awakened by warm water and find themselves surrounded by food—the simple sugars present in flour or added from elsewhere—they kick into metabolic overdrive. They have two primary ways to generate energy: with oxygen (aerobic respiration) and without it (anaerobic respiration, or fermentation). In the dense, oxygen-poor environment of a dough, fermentation dominates. The yeast's goal is not to make bread for us; it is simply to survive and multiply. Its waste products from this frantic quest for energy are ethanol (alcohol) and, most importantly for the baker, carbon dioxide gas.
The production of CO₂ is only half the story. A bowl of soupy batter would simply allow these tiny bubbles to escape, fizzing away into the air with nothing to show for it. The true genius of bread-making lies in the second key component: gluten. When wheat flour is hydrated and manipulated through mixing or kneading, two proteins—glutenin and gliadin—link together to form an intricate, elastic network known as gluten. Think of this network as billions of microscopic balloons. It is strong yet stretchy, capable of expanding under pressure. As the yeast cells metabolize and release CO₂, these gases become trapped within this vast, proteinaceous web of gluten. Each tiny bubble of carbon dioxide inflates its individual gluten compartment, causing the entire mass of dough to swell and rise, a process aptly named proofing.
This rising action is not a continuous, smooth process. It is a battle between creation and destruction. The yeast is constantly producing gas, inflating the gluten network. Simultaneously, the gluten strands, under tension, want to relax and contract, and the gas within seeks to escape. The baker's role is to manage this tension. The first rise, or bulk fermentation, allows the yeast population to expand and produce a vast amount of gas, creating the foundational air pockets. The dough is then gently punched down or folded. This isn't a punitive measure; it is a crucial reset. It expels the large, inefficient bubbles of carbon dioxide (along with some of the accumulated alcohol) and redistributes the yeast and temperature evenly throughout the dough. It also aligns and strengthens the gluten strands, preparing them for their final, spectacular expansion.
The shaped loaf then undergoes a final proof. Here, the yeast gets back to work for one last gaseous surge, inflating the dough to its optimal volume and creating the final crumb structure. Yet, this delicate aerated structure is still temporary and fragile. The gluten balloons are still just that—balloons. They can be deflated with a careless touch or a sudden draft. They require permanence. This is where the oven's heat performs the final act of the drama. As the temperature of the dough skyrockets, several transformative events occur in rapid succession. The yeast, in a last frenzied burst of activity, produces a final surge of carbon dioxide before the heat becomes too great and it perishes. The trapped gas bubbles expand even further due to the physics of heat (Charles's Law). The ethanol and other volatile compounds evaporate, contributing to the complex aroma of baking bread.
Most critically, the heat causes a process called coagulation. The starches in the flour gelatinize, absorbing water and swelling to form a stable gel that sets the loaf's structure. At around 80°C (176°F), the gluten proteins themselves coagulate or solidify. They permanently set into the expanded, inflated shape they achieved during proofing and the early stages of baking. The fleeting network of gas-filled balloons is transformed into a fixed, spongy matrix of solid foam. The windows of air that were once just pockets of CO₂ are now the alveoli of the finished loaf, forever captured. The water in the dough turns to steam, which also contributes a small but significant amount of additional leavening force, helping to achieve the coveted "oven spring"—the final rapid expansion of the loaf just before the crust sets.
The entire journey, from a sticky, dense mass to a light, fragrant loaf, is a testament to biological and chemical synergy. It is a process built upon the invisible exhalation of a microorganism. Each slice of bread is a fossil record of this activity; the pattern of holes and tunnels maps the precise history of the yeast's respiration and the gluten's capture of its gaseous output. The crusty exterior, the soft interior, the tearable crumb—none of it would exist without the relentless, life-giving production of carbon dioxide from Saccharomyces cerevisiae. It is a fundamental partnership between human, microbe, and molecule, resulting in one of humanity's oldest and most cherished staples. So the next time you break apart a warm piece of bread, remember that you are not just enjoying flour and water; you are savoring the captured breath of yeast.

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025